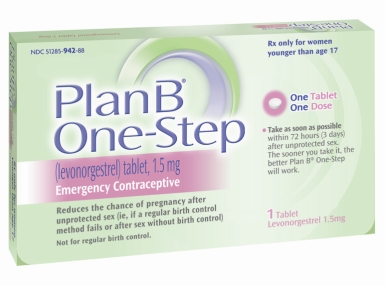
A federal judge has ordered that the Food and Drug Administration (FDA) must make the “morning-after” pill available over the counter to females of all ages, going against the initial requirement of having a prescription for girls under 17, according to published reports.
U.S. District Judge Edward R. Korman issued the decision after a lawsuit was filed by advocates of “reproductive-rights” groups that have been fighting to get rid of the age restrictions on the emergency contraception pill, reported Reuters.
“More than twelve years have passed since the Citizen Petition was filed and eight years since this lawsuit commenced. The FDA has engaged in intolerable delays in processing the petition. Indeed, it could accurately be described as an administrative agency filibuster,” the judge wrote in his ruling.
The pill, known as Plan B, is currently sold to women 17 or older without a prescription but who must show proper identification. If taken up to 72 hours after unprotected sex, Plan B can terminate a pregnancy.