BY DEENA BEASLEY
U.S. hospitals desperate to help very sick patients with COVID-19, the highly contagious respiratory disease caused by the new coronavirus, are trying a treatment first used in the 1890s that relies on blood plasma donated by recovered patients.
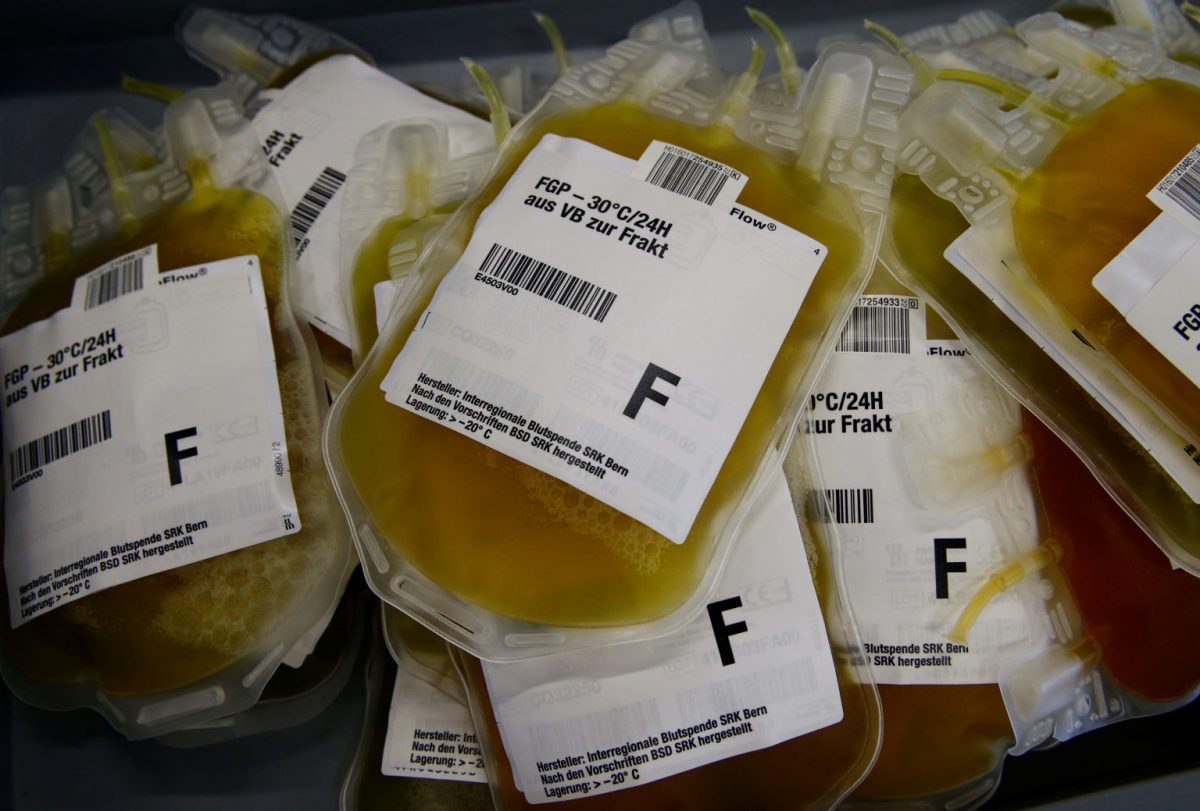
People who survive an infectious disease like COVID-19 are generally left with blood containing antibodies, or proteins made by the body’s immune system to fight off a virus. The blood component that carries the antibodies can be collected and given to newly infected patients – it is known as “convalescent plasma.”
More than a million Americans have tested positive for COVID-19, and epidemiologists say hundreds of thousands more likely have the disease.
To help match donors to hospitals, the AABB, formerly the American Association of Blood Banks, this week issued guidelines on plasma collection. The American Red Cross also launched an online registry for potential donors.
The U.S. Food and Drug Administration on Friday announced an “expanded access” program for convalescent plasma, coordinated by the Mayo Clinic in Rochester, Minnesota, aimed at making it easier for hospitals across the country to collect and use plasma.
Is there evidence this will work?
“Historically, this has worked,” said Dr. Jeffrey Henderson, associate professor of medicine and molecular microbiology at Washington University School of Medicine in St. Louis. “Before we had vaccines, this was used for infectious diseases like measles and diphtheria.”
Convalescent plasma was also successfully used during the 1918 flu pandemic, he said.
Doctors say protocols, such as dosage, are still uncertain for COVID-19 patients, but they believe the method is worth trying, at least until an effective COVID-19 vaccine or treatment is developed.
The Mayo Clinic and other U.S. sites are conducting a clinical study. Similar trials are under way in other countries where the virus has hit and some data has begun to emerge.
In one trial in China, levels of the virus in five seriously ill COVID-19 patients were undetectable after plasma transfusions, according to study results published last week in The Journal of the American Medical Association.
How is plasma being tried?
The process involves drawing blood from a donor – in this case someone who has recovered from COVID-19 but is in generally good health and meets other criteria for blood donation – and running it through a machine to extract the plasma. The remaining blood goes back into the donor.
The process takes up to 90 minutes, and plasma from a single donor can be used to treat three or four patients.
Donors must have been diagnosed with COVID-19 and need to wait a defined period of time after they test negative for the disease before donating plasma. Tests are also being developed to measure antibody volume.
Centers including Houston Methodist Hospital and several hospitals in hard-hit New York City have used the experimental treatment on an emergency basis for patients who are seriously ill with COVID-19.
Dr. Timothy Byun, a hematologist/oncologist at St. Joseph Hospital in Orange, California, dosed his first COVID-19 patient on Wednesday. He said the patient was doing better, but it was too early to tell if the therapy was effective.
St. Joseph, a 450-bed hospital, does not have a blood donation center and instead had to modify a dialysis machine to collect plasma from the donor.
Before the plasma infusion, Byun’s patient had received multiple treatments, including the malaria drug hydroxychloroquine and the intravenous anti-inflammatory drug Actemra, but his condition still worsened.
Risks of the plasma therapy could include infusion site reactions or other rare, allergic reactions.
“Of the current therapeutic options, I believe convalescent plasma offers the best chance of efficacy in treatment,” said Dr. Daniel McQuillen, an infectious disease specialist at Lahey Hospital & Medical Center in Burlington, Massachusetts.